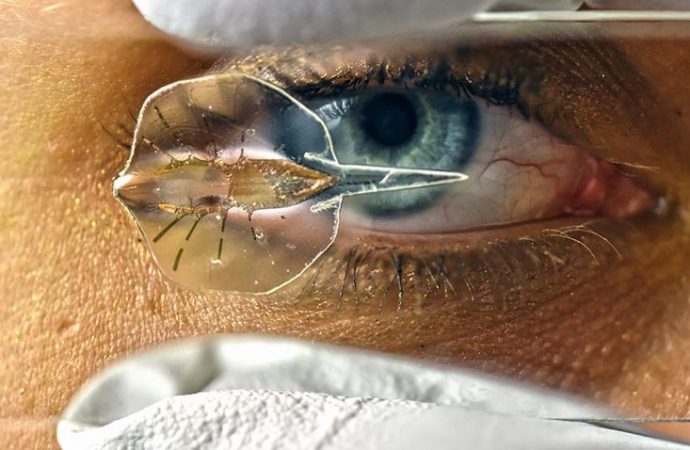
Part machine and part living cells, this artificial stingray can be maneuvered through obstacle courses.

Photo: Karaghen Hudson and Michael Rosnach
Kevin Kit Parker wants to build a human heart. His young daughter loves the New England Aquarium in Boston. In this Science report, father’s and daughter’s obsessions have combined in an unlikely creation: a nickel-sized artificial stingray whose swimming is guided by light and powered by rat heart muscle cells.
Incorporating advances in engineering, cell culture, genetics, and biomechanics, the “living” robot is “clearly a technical tour de force,” says Adam Summers, an integrative biologist at the University of Washington, Seattle. And some think that by melding cells and artificial materials into a pulsating structure, the device brings Parker’s dream of engineering a human heart a step closer. “One can imagine that one day we can use this technology to rebuild parts of the human body,” says Kedi Xu, a neural engineer at Zhejiang University in Hangzhou, China.
Parker, an applied physicist at Harvard University, made his first foray into robotics 5 years ago after he was captivated by the jellyfish during a visit to the aquarium. The creature’s rhythmic pumping reminded him of the beating heart. His team had already gotten heart muscle cells to grow into thin films on silicone, and he wondered whether he could put the cells to work by incorporating them into a jellyfishlike “pump.”
The result was a “medusoid”—a simple artificial creature composed of heart muscle cells overlaid on a sheet of silicone molded into a shallow cup rimmed with flaps. A bath of salt-sugar solution sustained the cells, and tiny jolts of electricity made the cells contract, changing the shape of the silicone cup so that the “jellyfish” expelled liquid, propelling it through its bath. “For me this was just a training exercise,” Parker recalls. “I’m trying to get better and better at building muscular pumps.”
His new effort was also inspired by a visit to the aquarium. When his daughter touched a stingray in the petting tank, it flicked one side of its body and veered away. “Maybe there is something similar with how the stingray changes direction and heart flow,” Parker thought. So he decided to move up the tree of life, from jellyfish to rays, and increase the complexity of his team’s “biohybrids.”

Kevin Kit Parker and his daughter compare a ray robot on a slide with a skate.
Karaghen Hudson
Again thanks to his daughter, he envisioned a simple way to control the new robot: light. When she was a toddler, Parker would guide her down the sidewalk by shining his laser pointer on the ground and having her stomp on the light. Perhaps his team could do something similar in an artificial ray by making the muscle cells contract in response to light. They turned to optogenetics, in which cells are genetically endowed with light-responsive molecules that trigger signaling cascades. Parker’s team had no experience with the method, but with a bravado befitting a lieutenant colonel who fought in Afghanistan before and after joining Harvard’s faculty, he decided to go ahead—cobbling together funding from the Army, the National Institutes of Health, and others. Parker asked a new postdoc, Sung-Jin Park, to oversee the work, confidently predicting the project would produce a paper featured on the cover of Science. “I thought it was crazy … impossible,” Park recalls.
It took 4 years to fulfill Parker’s prediction. Park and others began by taking apart stingrays to learn how the muscles were arranged; later, colleagues in another lab analyzed how the muscles drive the fins in the synchronized undulations that propel the ray. To mimic the animal’s basic anatomy, Park experimented with many soft-robot configurations, eventually settling on a multipronged gold skeleton sandwiched between two silicone layers.
Animating each ray are about 200,000 heart cells harvested from 2-day-old rat embryos and placed on top of the silicone. The silicone bears a template consisting of an extracellular protein, fibronectin, which guides the growth of the cells into a radiating pattern similar to the muscles in the real ray. Getting this architecture right was critical to making cardiac cells do the work of skeletal muscles, says Andrew McCulloch, a bioengineer at the University of California, San Diego, who was not involved with the work.
But Parker’s group didn’t follow the ray’s muscle structure exactly. “They took a shortcut,” says Frank Fish, a biomechanist at West Chester University in Pennsylvania. Real rays have two sets of muscles within each pectoral fin, pulling in opposite directions to move a fin down, then up. The ray robot has just one set of muscles, which bend the fins downward; the spring action of the gold skeleton pulls the fins back up.
Infected with a virus that delivers the gene encoding the optogenetic molecular switch, the modified cardiac cells twitch when blue light shines on them. But translating that effect into coherent motion took months of tweaking; simply getting a robot ray to move forward when light stimulated the front of its fin took Park 200 tries. Ultimately, he built 100 more robots and showed they could navigate underwater obstacle courses. To negotiate turns, Park guides a ray with two light sources, one pointed at each fin. Changing the frequency of the light slows or speeds up the contraction rate; by making one side beat faster than the other, he steers the robot left or right.
The rays move only about 9 meters per hour and turn slowly—quite pathetic by real stingray standards. Even so, “putting it all together is remarkable,” says Alexander Smits, a mechanical engineer at Princeton University. Fish, who helped design larger “manta bots” with silicone fins flapped by electronic-controlled rods and cables, calls the rays “a major leap forward in terms of robotics.” He adds, “we’re getting to the point where there really is a fusion between biology and engineering.”

Read more about how the robotic stingray was captured for this week’s cover of Science.
Ken Richardson
There’s a long way to go, however. Live-muscle robots work only in nutrient-filled solutions warmed to a rat’s body temperature; keeping them going in a more natural environment will be a challenge, Fish says.
And it’s unclear whether the approach will lead to practical robots or to Park and Parker’s true interest: a bioartificial heart. Simply putting down a second layer of muscle cells is daunting, nevermind recapturing the full complexity of the heart or a complete animal. The ray robot “is not really particularly related to anything in the heart,” especially because the heart muscle cells are “being used in a fairly unnatural way,” says Denis Buxton, a program officer at the National Heart, Lung, and Blood Institute in Bethesda, Maryland.
But to Parker and others that “unnatural way” is very informative. “The heart is a hollow muscle,” explains Simon Hoerstrup, a cardiovascular surgeon at the University of Zurich Institute for Regenerative Medicine in Switzerland. “Many of the features you see in this ray, you find in the heart.”
Parker regards his team’s miniature ray robot as a piece of art as well as technology: “Everyone is going to see something different” in it, he says. “I’m looking at it and I’m trying to understand the heart—and impress my 7-year-old daughter.”































Leave a Comment
You must be logged in to post a comment.