A novel system developed by researchers at the Massachusetts Institute of Technology (MIT) could make it possible to control the way a liquid moves over a surface, using only visible light.
“We were inspired by the work in photovoltaics, where dye sensitization was used to improve the efficiency of absorption of solar radiation,” said Kripa Varanasi, an associate professor of mechanical engineering at MIT, and senior author of a paper describing the new system in the journal Nature Communications.
The team’s initial goal was to find ways of separating oil from water, for example, to treat the frothy mixture of briny water and crude oil produced from certain oil wells.
Sometimes electrostatic methods are used, but these are energy-intensive and don’t work when the water is highly saline, as is often the case. Instead, Dr. Varanasi and co-authors explored the use of ‘photoresponsive’ surfaces, whose responses to water can be altered by exposure to light.
By creating surfaces whose interactions with water — a property known as wettability — could be activated by light, the authors found they could directly separate the oil from the water by causing individual droplets of water to coalesce and spread across the surface. The more the water droplets fuse together, the more they separate from the oil.
Photoresponsive materials have been widely studied and used; one example is the active ingredient in most sunscreens — titanium dioxide (TiO2).
But most of these materials, including titanium dioxide, respond primarily to UV light and hardly at all to visible light. Yet only about 5% of sunlight is in the UV range.
So the researchers figured out a way to treat the titanium dioxide surface to make it responsive to visible light. They did so by first using a layer-by-layer deposition technique to build up a film of polymer-bound titanium dioxide particles on a layer of glass.
Then the team dip-coated the material with a simple organic dye. The resulting surface turned out to be highly responsive to visible light, producing a change in wettability when exposed to sunlight that is much greater than that of the titanium dioxide itself.
When activated by sunlight, the material proved very effective at ‘demulsifying’ the oil-water mixture — getting the water and oil to separate from each other.
“The coupling of the dye to titanium dioxide particles allows for the generation of charge carriers upon light illumination,” Dr. Varanasi said.
“This creates an electric potential difference to be established between the surface and the liquid upon illumination, and leads to a change in the wetting properties.”
“Saline water spreads out on our surface under illumination, but oil doesn’t,” added co-author Dr. Gibum Kwon, an assistant professor at the University of Kansas.
“We found that virtually all the seawater will spread out on the surface and get separated from crude oil, under visible light.”
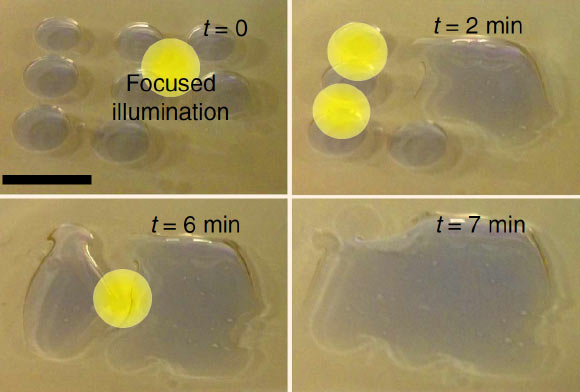
The same effect could also be used to drive droplets of water across a surface, as the team demonstrated in a series of experiments.
By selectively changing the material’s wettability using a moving beam of light, a droplet can be directed toward the more wettable area, propelling it in any desired direction with great precision. Such systems could be designed to make microfluidic devices without built-in boundaries or structures. The movement of liquid — for example a blood sample in a diagnostic lab-on-a-chip — would be entirely controlled by the pattern of illumination being projected onto it.
“By systematically studying the relationship between the energy levels of the dye and the wettability of the contacting liquid, we have come up with a framework for the design of these light-guided liquid manipulation systems,” Dr. Varanasi said.
“By choosing the right kind of dye, we can create a significant change in droplet dynamics. It’s light-induced motion — a touchless motion of droplets.”
The switchable wettability of these surfaces has another benefit — they can be largely self-cleaning. When the surface is switched from water-attracting to water-repelling, any water on the surface gets driven off, carrying with it any contaminants that may have built up.
Source: Sci News































Leave a Comment
You must be logged in to post a comment.